HEALTH/WELLNESS: Reopens Carlsbad Facility to Meet Growing Capacity Needs
BY KAREN PEARLMAN
APRIL 16, 2024
CARLSBAD – More than four decades after its founding, supplement manufacturer Natural Alternatives International, Inc. (Nasdaq: NAII) continues to bring good health to the masses.
NAI is a leading formulator, manufacturer and marketer of nutritional supplements and provides strategic partnering services to several hundred clients. It has plants in Carlsbad, Vista and Switzerland.
NAI’s partnership approach offers myriad innovative nutritional and food products and services to clients including scientific research, proprietary ingredients, customer-specific nutritional or food product formulation, product testing and evaluation, marketing management and support, packaging and delivery system design, regulatory review and international product registration assistance.
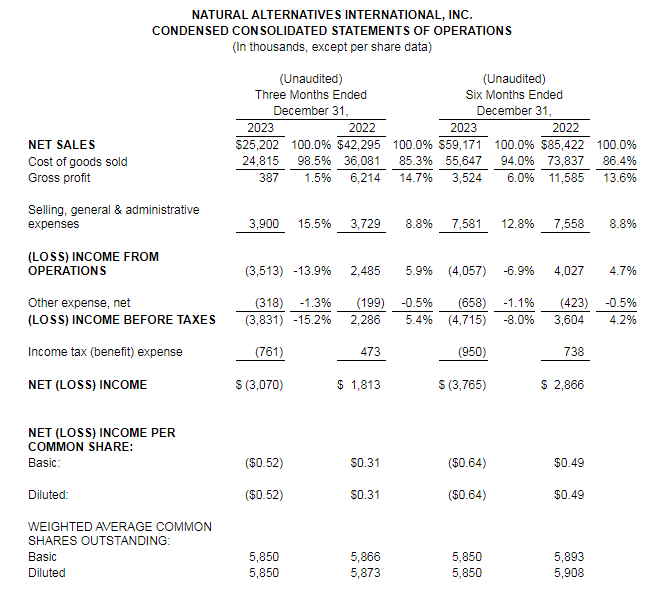
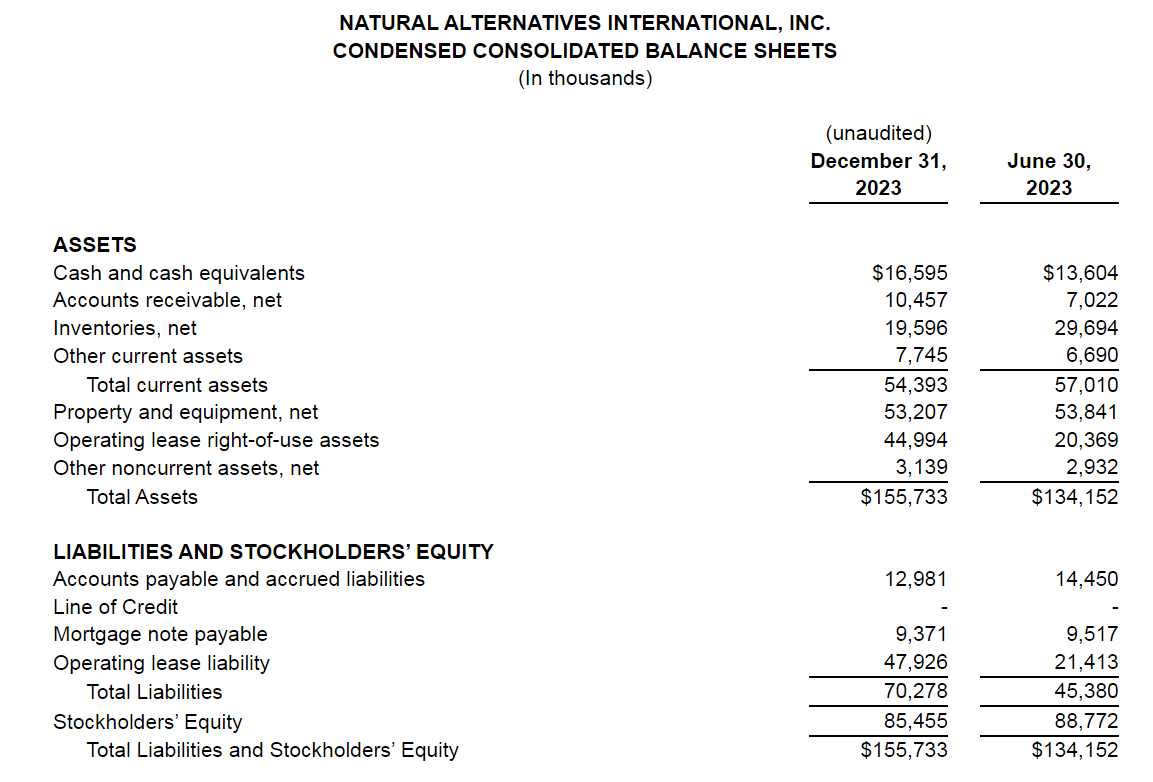
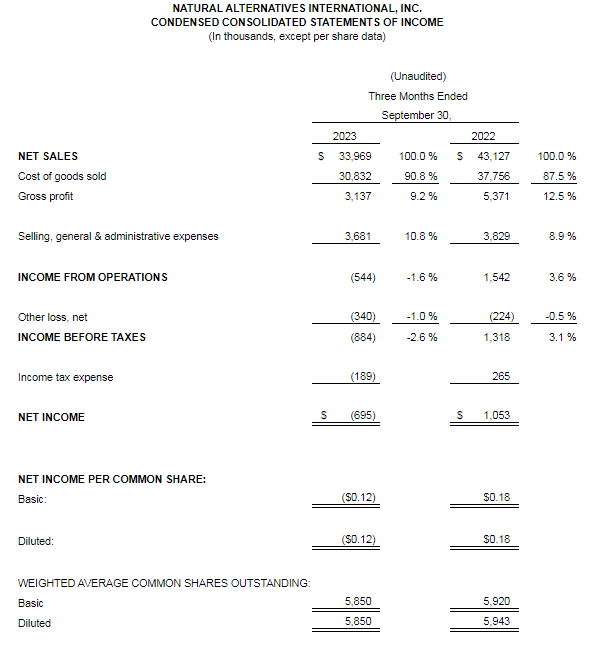
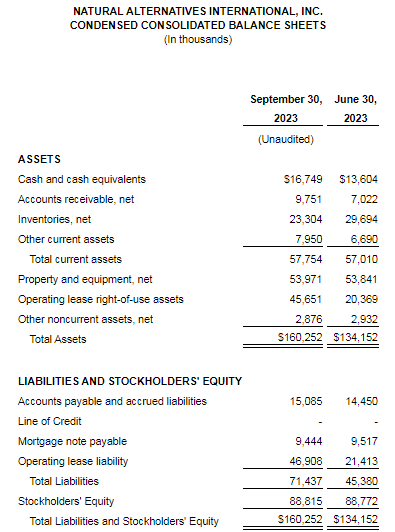
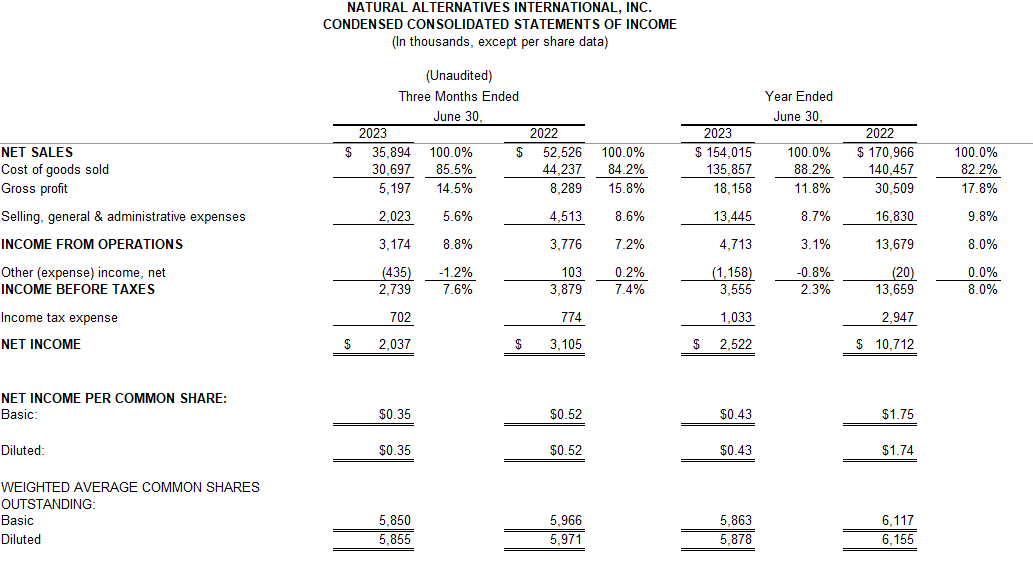
And while the company has seen success – to the tune of $154 million in revenue in 2023 – some of the customers it partners with have had challenges. Sales have grown considerably over the last 40 years but not always on a consistent basis, said NAI President and COO Ken Wolf.
“Our business is highly contingent on the success of our customers’ businesses in terms of product acceptance, new product launches, marketing plans, etc.,” Wolf said.
Last year, NAI closed its high-volume powder blending and packaging facility in Carlsbad after a little more than two years in service because one of its baseline customers, a private party which the company does not share, was overstocked. But with the customer’s previous over-inventory situation now resolved, NAI is going to re-open the plant in May.
“Restarting our facility is a direct result of our growing capacity needs,” said Mark LeDoux, NAI founder, chairman and CEO. “Powder packaged delivery systems are a growing segment within our industry and this plant can provide an annual capacity of over 26 million pounds of high-quality blended powder products.”
NAI purchased the Carlsbad facility for $35 million in August 2021 and spent more than 20 months retrofitting the space with a heavy focus on efficient high-volume powder production and gusseted bag packaging.
NAI’s Carlsbad facility had 88 workers, 28 of whom were reassigned within the company. Reactivating the facility will require employee recruitment of approximately 60 people, Wolf said.
The Prescriptive Beginning
LeDoux founded NAI in 1980, with assistance from his parents.
“The intent was to utilize seed capital, which was under $100,000 to secure product from contract manufacturers and to present these products to practitioners and to natural food stores which were nascent in the 1980s,” LeDoux said.
He said he started the company after viewing firsthand as a scrub-tech in a teaching hospital “the ravages of poor choices reflected in the need for surgical intervention.”
“Spending considerable time and effort researching the food supply and consumer behaviors it became apparent that in America, the population was probably the most overfed, but undernourished demographic in the western world,” LeDoux said.
“Corporate farming has led to increases in production, but not necessarily increases in the nutrient density of essential foods, thereby creating deficits in the choices consumers have to select from.”
LeDoux said that while drugs have their place in the medical arsenal, he wanted to focus on identifying natural alternatives to synthesized chemical analogs.
He discovered scientific literature extolling the need for higher quality foods, improved farming techniques, reductions in the use of dangerous pesticides and herbicides and enhancing the world “through the best of nutrition prior to resorting to treating symptoms versus root causes of diseases.”
“Early on, NAI products took root in the health food store channel and were enthusiastically supported by consumers based on the fact that they worked,” LeDoux said.
As contract manufacturers could not keep up with the demand growth, LeDoux and his family decided to purchase manufacturing equipment, located a small industrial building in San Marcos near a UPS distribution center and began production of its own products.
One of NAI’s early customers was Jenny Craig, which needed supplements designed for their customers to complement the low-calorie diets Craig extolled.
NASDAQ and Expansion Across the Pond
NAI started trading publicly on Nasdaq in 1997, left for a short time to list on the American Stock Exchange, but LeDoux said the company “did a 50:1 reverse split and ultimately transitioned to the Nasdaq national market where it resides today.”
After nearly two decades of NAI in Southern California, LeDoux opened a facility in Lugano, Switzerland in 1999.
That facility helped customers expand their businesses internationally and provided NAI with the ability to attract new international customers. NAI products are now distributed in more than 40 countries.
“In general, manufacturing products in Switzerland for use in the EU saves our customers approximately 15% on the cost of their products due to the savings in Duty and Freight costs,” Wolf said.
NAI for more than a decade has also manufactured and distributed CarnoSyn, a patented beta-alanine royalty supplement. The supplement has been studied and found to support an increase in muscle carnosine and athletic performance and a contributor to overall brain and muscle health, promoting healthy aging.
NAI reported last year that CarnoSyn sales revenue increased 36.1% to $3.6 million during the fourth quarter of fiscal year 2023, as compared to $2.6 million for the fourth quarter of fiscal year 2022.
COVID and Other Challenges
While COVID challenged the company internally, with protocols for facility workers ever changing in a time NAI was considered an essential business, the pandemic also brought more revenue through increased need by NAI’s partners to fill an uptick in their customers’ supplements.
COVID did bring what LeDoux called “a dysfunctional supply chain, and a surge in demand for supplements during the pandemic,” but that the pandemic was instructive on many levels “and we survived and prospered.”
LeDoux said NAI weathered other external challenges twice in the 1980s.
The first time was during a medicine tampering incident in 1982, when seven people died after taking cyanide-laced Tylenol.
“(It was) significant in that the use of capsules to provide supplements or pharmaceuticals was called into question, whereas the ultimate concern was on package safety and chain of custody protection,” he said.
Then in 1989, there was a Tryptophan issue, LeDoux recalled, where a Japanese multi-national vendor took short-cut steps in the fermentation of their Tryptophan molecule, bringing in the Centers for Disease Control and Prevention and the Food and Drug Administration.
“NAI was instrumental in helping the CDC and FDA identify the Peak E component of the improperly filtered Tryptophan from Japan,” he said.
Natural Alternatives International, Inc.
FOUNDED: 1980
FOUNDER AND CEO: Mark LeDoux
HEADQUARTERS: Carlsbad, Vista and Lugano, Switzerland
BUSINESS: Health/Manufacturing
REVENUE: $154 million (2023)
STOCK: NAII (Nasdaq)
EMPLOYEES: 250
WEBSITE: nai-online.com
CONTACT: 760-736-7700
SOCIAL IMPACT: NAI fosters leadership, respect, and integrity as fundamental traits of its personal and corporate culture. The company values diversity, employee engagement and ethical and environmental responsibility.
NOTABLE: NAI’s core mission is to support its clients and advance responsible nutrition on a global scale, achieving this through dedication to excellence through extending beyond its walls to ensure lasting partnerships with clients, employees, vendors, shareholders and the environment.